 Image 1 of 3
Image 1 of 3

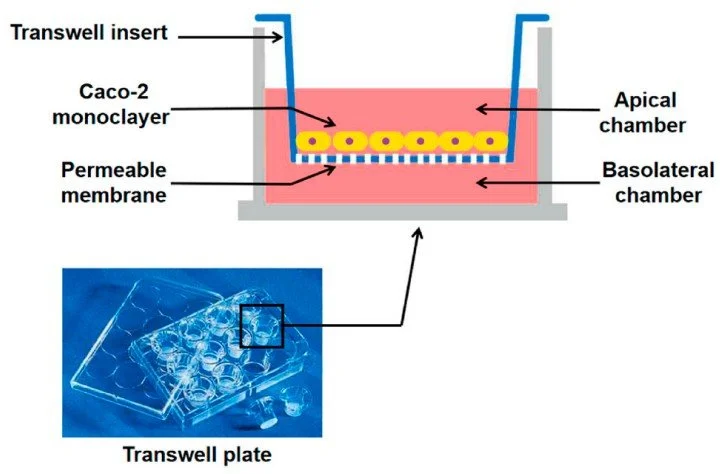 Image 2 of 3
Image 2 of 3

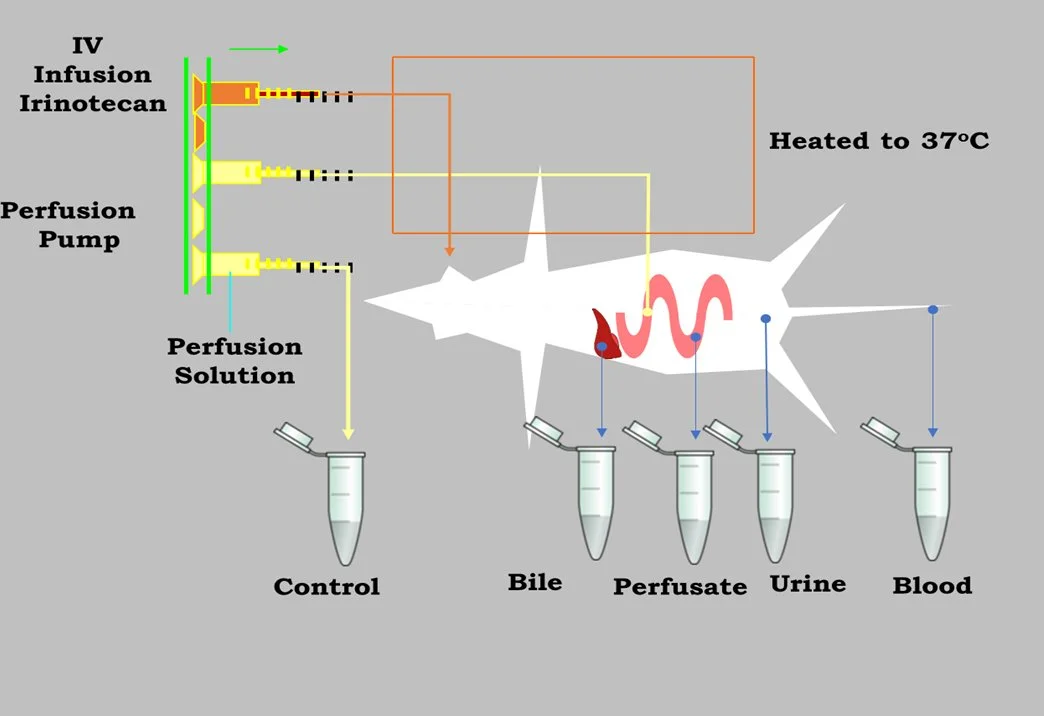 Image 3 of 3
Image 3 of 3




Permeability and transporter identification
Services: Permeability determination, efflux ratio, transporter identification, transporter inhibition
Models: Caco-2 cell culture mode, rat intestinal perfusion model
The Caco-2 cell line is a vital in vitro model of the human intestine, extensively utilized for predicting drug absorption and permeability. Recognized by the FDA as a valid model within the Biopharmaceutics Classification System (BCS), Caco-2 cells play a crucial role in developing new drug applications and understanding drug interactions in the gastrointestinal tract. These cells are integral to permeability assays, allowing researchers to evaluate how effectively drugs can traverse the intestinal barrier, which is essential for assessing the potential of oral drug delivery. The use of Caco-2 cells is further endorsed by both the FDA and the European Medicines Agency (EMA), as their permeability data can accurately predict human gastrointestinal absorption.
Efflux transporters, including P-glycoprotein (P-gp), breast cancer resistance protein (BCRP), and multidrug resistance-associated proteins (MRPs), play a crucial role in reducing drug bioavailability. They achieve this by actively pumping substrates back into the intestinal lumen, thereby limiting systemic absorption. The Caco-2 cell culture model, derived from human intestinal epithelial cells, is an essential tool for identifying transporter-mediated drug interactions due to its expression of multiple efflux transporters and its ability to form polarized monolayers with tight junctions.
The intestinal perfusion model in rodents is widely used to study drug permeability and predict human intestinal absorption. This in situ model involves perfusing drug solutions through one or more specific intestinal regions (e.g., duodenum, jejunum, ileum, colon) while measuring effective permeability coefficients and drug absorption percentage. Numerous studies have demonstrated strong correlations between P<sub>eff</sub> values and human intestinal permeability, enabling predictions of oral drug absorption fractions. The results from intestinal perfusion studies are particularly valuable for evaluating regional intestinal differences and the effects of permeation enhancers, supporting drug development for modified-release formulations.
At XLog, we provide excellent service through our Caco-2 cell culture model, intestinal perfusion studies, determining absorption in the gastrointestinal tract using both rats and mice. Our reliable and fast turnaround ensures that clients receive high-quality data to advance their drug development projects effectively.
Additionally, we utilize specific transporter inhibitors and siRNA-mediated gene silencing methods to identify the involved transporters. This approach allows us to investigate the mechanisms underlying poor bioavailability and efficacy, as well as potential drug-drug interactions via transporters, in alignment with regulatory guidelines.
Services: Permeability determination, efflux ratio, transporter identification, transporter inhibition
Models: Caco-2 cell culture mode, rat intestinal perfusion model
The Caco-2 cell line is a vital in vitro model of the human intestine, extensively utilized for predicting drug absorption and permeability. Recognized by the FDA as a valid model within the Biopharmaceutics Classification System (BCS), Caco-2 cells play a crucial role in developing new drug applications and understanding drug interactions in the gastrointestinal tract. These cells are integral to permeability assays, allowing researchers to evaluate how effectively drugs can traverse the intestinal barrier, which is essential for assessing the potential of oral drug delivery. The use of Caco-2 cells is further endorsed by both the FDA and the European Medicines Agency (EMA), as their permeability data can accurately predict human gastrointestinal absorption.
Efflux transporters, including P-glycoprotein (P-gp), breast cancer resistance protein (BCRP), and multidrug resistance-associated proteins (MRPs), play a crucial role in reducing drug bioavailability. They achieve this by actively pumping substrates back into the intestinal lumen, thereby limiting systemic absorption. The Caco-2 cell culture model, derived from human intestinal epithelial cells, is an essential tool for identifying transporter-mediated drug interactions due to its expression of multiple efflux transporters and its ability to form polarized monolayers with tight junctions.
The intestinal perfusion model in rodents is widely used to study drug permeability and predict human intestinal absorption. This in situ model involves perfusing drug solutions through one or more specific intestinal regions (e.g., duodenum, jejunum, ileum, colon) while measuring effective permeability coefficients and drug absorption percentage. Numerous studies have demonstrated strong correlations between P<sub>eff</sub> values and human intestinal permeability, enabling predictions of oral drug absorption fractions. The results from intestinal perfusion studies are particularly valuable for evaluating regional intestinal differences and the effects of permeation enhancers, supporting drug development for modified-release formulations.
At XLog, we provide excellent service through our Caco-2 cell culture model, intestinal perfusion studies, determining absorption in the gastrointestinal tract using both rats and mice. Our reliable and fast turnaround ensures that clients receive high-quality data to advance their drug development projects effectively.
Additionally, we utilize specific transporter inhibitors and siRNA-mediated gene silencing methods to identify the involved transporters. This approach allows us to investigate the mechanisms underlying poor bioavailability and efficacy, as well as potential drug-drug interactions via transporters, in alignment with regulatory guidelines.
